√70以上 heterogeneous catalyst vs homogeneous catalyst 121521
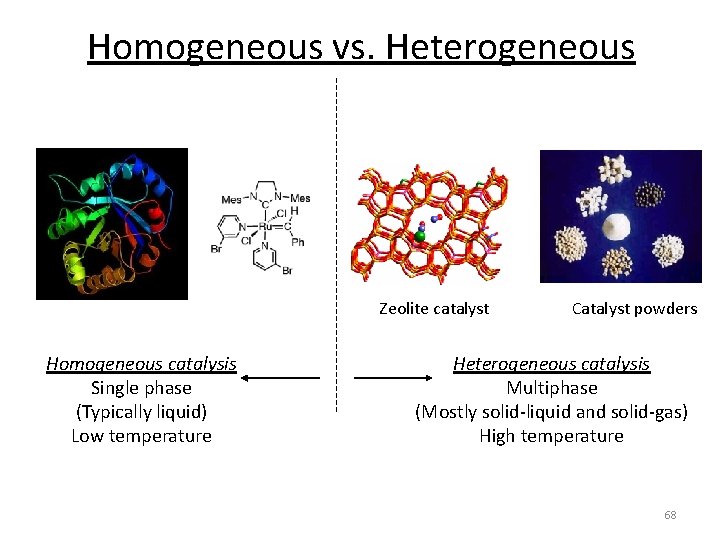
Report 5 years ago #2 Homogeneous means that the catalyst and the products/reactants are in the same chemical state or phases (solid/liquid/gas) Heterogeneous means that the catalyst and the products/reactants are in different chemical states or phases Look at the information given in the question and you should figure this out cHomogeneous catalysts are those that occupy the same phase as the reaction mixture (typically liquid or gas), while heterogeneous catalysts occupy a different phase Generally, heterogeneous catalysts are solid compounds that are added to liquid or gas reaction mixtures Catalysts are conventionally divided into two categories homogeneous and heterogeneous Enzymes, natural biological catalysts, are often included in the former group, but because they share some properties of both but exhibit some very special properties of their own, we will treat them here as a third category
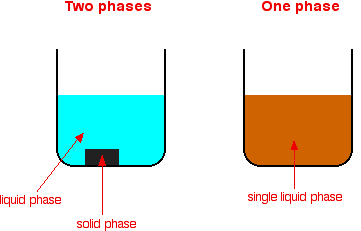
Types Of Catalysis
Heterogeneous catalyst vs homogeneous catalyst
Heterogeneous catalyst vs homogeneous catalyst- Explanation There are two types of catalysts, homogeneous and heterogeneous A homogeneous catalyst is a catalyst that is in the same phase as the reactants, while a heterogeneous catalyst is in a different phase than the reactants Classify the following as homogeneous or heterogeneous catalysis and present your reasoning (a) The increased rate in the presence of NO(g) of SO 2 (g) oxidation by O 2 (g) to SO 3 (g) (b) The hydrogenation of liquid vegetable oil using a finely divided nickel catalyst (c) The conversion of an aqueous solution of Dglucose to a D,L mixture



Www Catalysis De Fileadmin User Upload Main Dateien 1 Forschung Fb Rosenthal Probevorlesung Hapke Hand Out Pdf
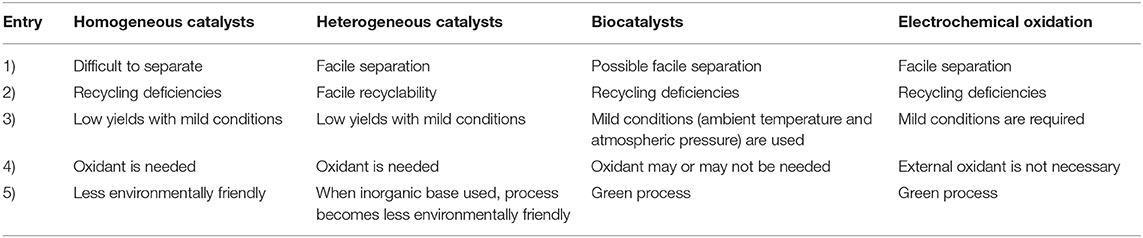
Heterogeneous catalysts minimize the problems of homogeneous catalysis in terms of catalyst regeneration and the possibility to be reused in continuous processes It is reported in the literature Crossing the divide between homogeneous and heterogeneous catalysis in water oxidation ron K Vannucci, Leila Alibabaei, Mark D Losego, Javier J Concepcion, Berç Kalanyan, Gregory N Parsons, and Thomas J Meyer a Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC ;Tel 31 46 b Department of Chemistry, Joseph Black Building, University of Glasgow, Glasgow, UK G12 8QQ
Heterogeneous catalysts are easier to recover Collision frequency is greater for homogeneous catalysts Homogeneous catalysts are often more sensitive to temperature Homogeneous catalysts are often more expensive This video provides a basic introduction into homogeneous and heterogeneous catalysts A Homogeneous catalyst exists in the same phase as the reactants and This is attributed to both catalyst binding mode and structural rigidity imparted by covalent grafting, which highlights how catalyst design has to be considered for the direct transposition of molecular catalysis on (photo)electrode surfaces
Herein, the first comparison of the mechanisms of glucosetofructose isomerization in aqueous media enabled by homogeneous (CrCl 3 and AlCl 3) and heterogeneous catalysts (Snbeta) by using isotopiclabeling studies is reportedA pronounced kinetic isotope effect (KIE) was observed if the deuterium label was at the C2 position, thus suggesting that a hydrogen shift Homogeneous catalysis as an important tool for organic synthesis (M Catellani) Photochemistry and photocatalysis (V Balzani) The discovery and evolution of metallocenebased olefin polymerization catalysts (V Kaminsky) Homogeneous and heterogeneous catalysis bridging the gap through surface organometallic chemistry (R Psaro)Homogeneous and heterogeneous catalysis in industry Johannes G de Vries a and S David Jackson b a DSM Innovative Synthesis BV, PO Box 18, 6160 MD Geleen, The Netherlands Email HansJGVriesde@dsmcom;
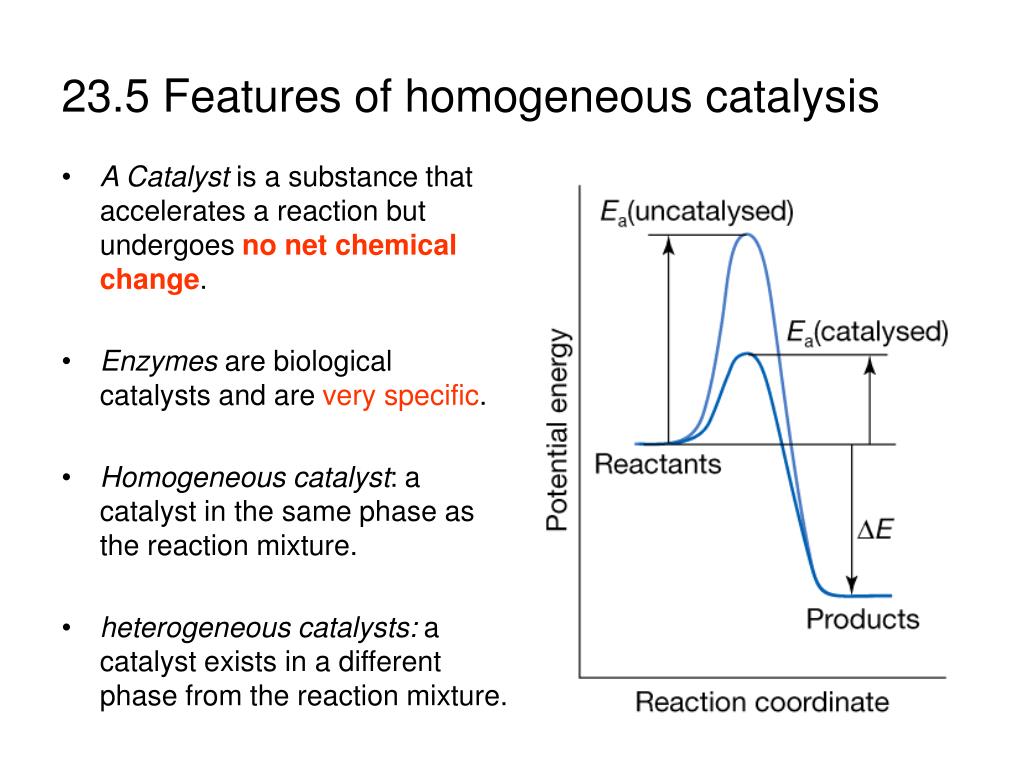



Ppt 23 5 Features Of Homogeneous Catalysis Powerpoint Presentation Free Download Id




Crossing The Divide Between Homogeneous And Heterogeneous Catalysis In Water Oxidation Pnas
Difference Between Homogeneous Catalysis and Heterogeneous Catalysis Video Lecture from Surface Chemistry Chapter of Chemistry Class 11 for HSC, IIT JEE, CBSA heterogeneous catalyst is a participant in a chemical reaction that is not part of the same phase of matter as the actual reactants For instance, liquids may undergo reaction in the presence of a solid catalyst While the catalyst speeds the process of a reaction, itHeterogeneous catalysts, on the other hand, lack the high tunability of homogeneous catalysts but have the advantages of being readily recyclable and easily adopted in a fixedbed flow reactor The robustness, stability and recyclability of heterogeneous catalysts under harsh conditions makes them ideal catalysts for cleavage of strong




Heterogeneous Catalysis Alchetron The Free Social Encyclopedia




Heterogeneous Vs Homogeneous Catalysts Advantages
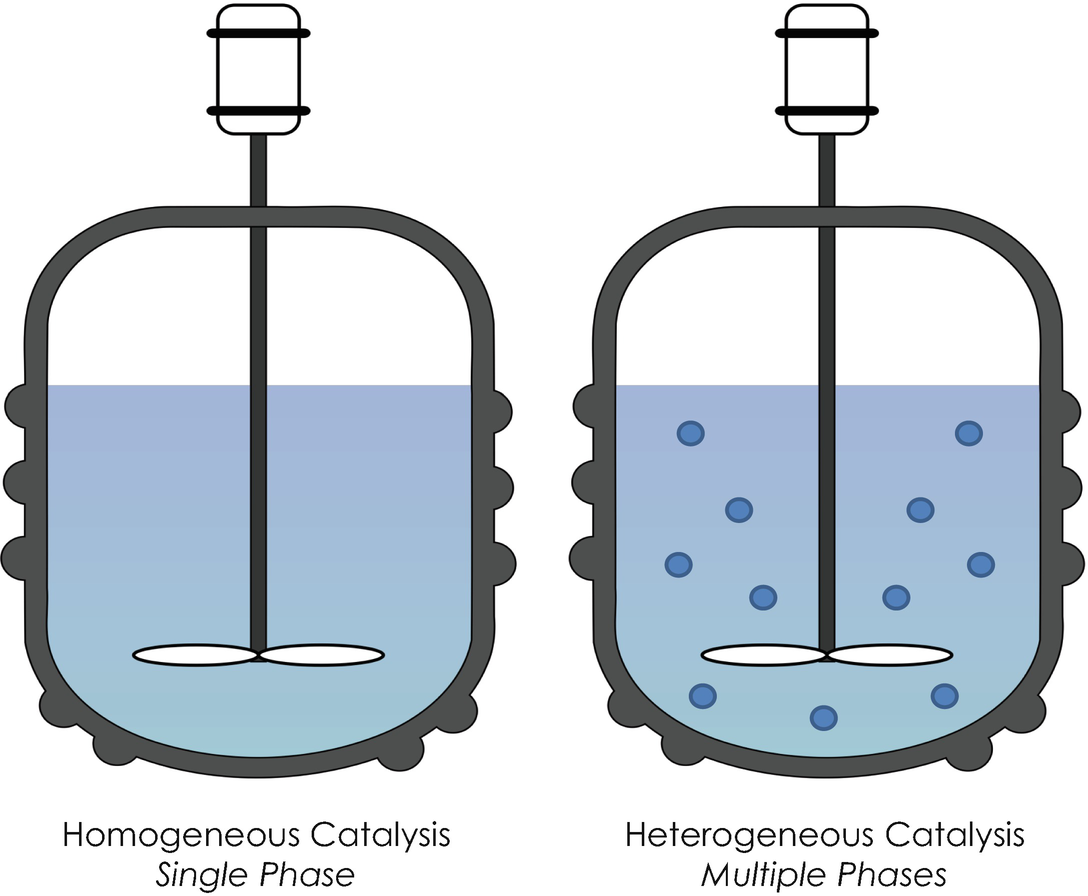
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or productsThe process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures (eg oil and water), or anywhereHeterogeneous versions of homogeneous catalysts can often be produced and may have certain advantages in use particularly on a commercial scale The catalytic chemistry of supported rhodium compounds for the carbonylation of methanol is described and the behaviour of heterogeneous and homogeneous operation compared in order to illustrate the relations between the two catalystSO using 3 NO as a homogeneous catalyst, which occurs in the gaseous phase On the other hand, most of the processes using homogeneous catalysts occur in a liquid phase whereas for the heterogeneous catalysts, the catalyst is usually in a solid form, and the reaction occurs either in the liquid or gaseous phase The fact that the catalysts is in a




Homogeneous Heterogeneous Catalysts 19 21 Cie As Chemistry Notes




Pdf Heterogeneous Catalytic Chemistry By Example Of Industrial Applications Semantic Scholar
Emulsion catalysis has been demonstrated to be quite useful strategy for overcoming the compatibility of different media in the liquid–liquid biphasic reaction system This chapter introduces the basic concept of emulsion catalysis and its application for the oxidative desulfurization, Lewis acidcatalyzed organic reactions, organocatalyticIn chemistry, homogeneous catalysis is catalysis in a solution by a soluble catalyst Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solidgas, respectively In this respect, heterogeneous singlemetalsite catalysts combine features of homogeneous and heterogeneous catalysis Ideally, in metalbased catalysts, any metal atom constitutes a single low




26 Homogeneous And Heterogeneous Catalysis Chapter




1 Homogeneous Vs Heterogeneous Catalysts Download Table
Homogeneous and heterogeneous catalysis Activity is the ability of the catalyst to accelerate a chemical reaction The degree can be as high as 100 times in certain reactions A catalytic cycle processes in which the reactant and catalyst undergo several transformations before making theHomogeneous versus Heterogeneous Catalysts The relative merits of homogeneous and heterogeneous catalysts to the industrial chemist are well known and are only outlined here Homogeneous catalysts have better de fined active sites, usually have all of the metal atoms available as the catalyst, and steric In order to verify the mode of catalysis by dynamic Pd nanoclusters, mercury poisoning tests were performed (Fig 3c) It is well established that heterogeneous catalysts, in contrast to homogenous




Difference Between Homogeneous And Heterogeneous Catalyst Compare The Difference Between Similar Terms



Www Ethz Ch Content Dam Ethz Special Interest Chab Icb Van Bokhoven Group Dam Coursework Catalysis 17 Homogeneous Heterogeoenous Catalysis Mesoporousmaterials Pdf
Homogeneous vs heterogeneous catalysis Dr habil Marko Hapke 3 3 Heterogeneous Catalysis Homogeneous Catalysis Catalyst and reactant(s) are in the same phase Catalyst and reactant(s) are in different phases Definitions General features Different reaction phases possible „classic"Homogeneous catalysts are those which exist in the same phase (gas or liquid) as the reactants, while heterogeneous catalysts are not in the same phase as the reactants Typically, heterogeneous catalysis involves the use of solid catalysts placed in a liquid reaction mixture Catalysis Note the lowered activation energy of the catalyzed pathwayNote It is important that you remember the difference between the two terms heterogeneous and homogeneous hetero implies different (as in heterosexual) Heterogeneous catalysis has the catalyst in a different phase from the reactants homo implies the same (as in homosexual) Homogeneous catalysis has the catalyst in the same phase as the reactants
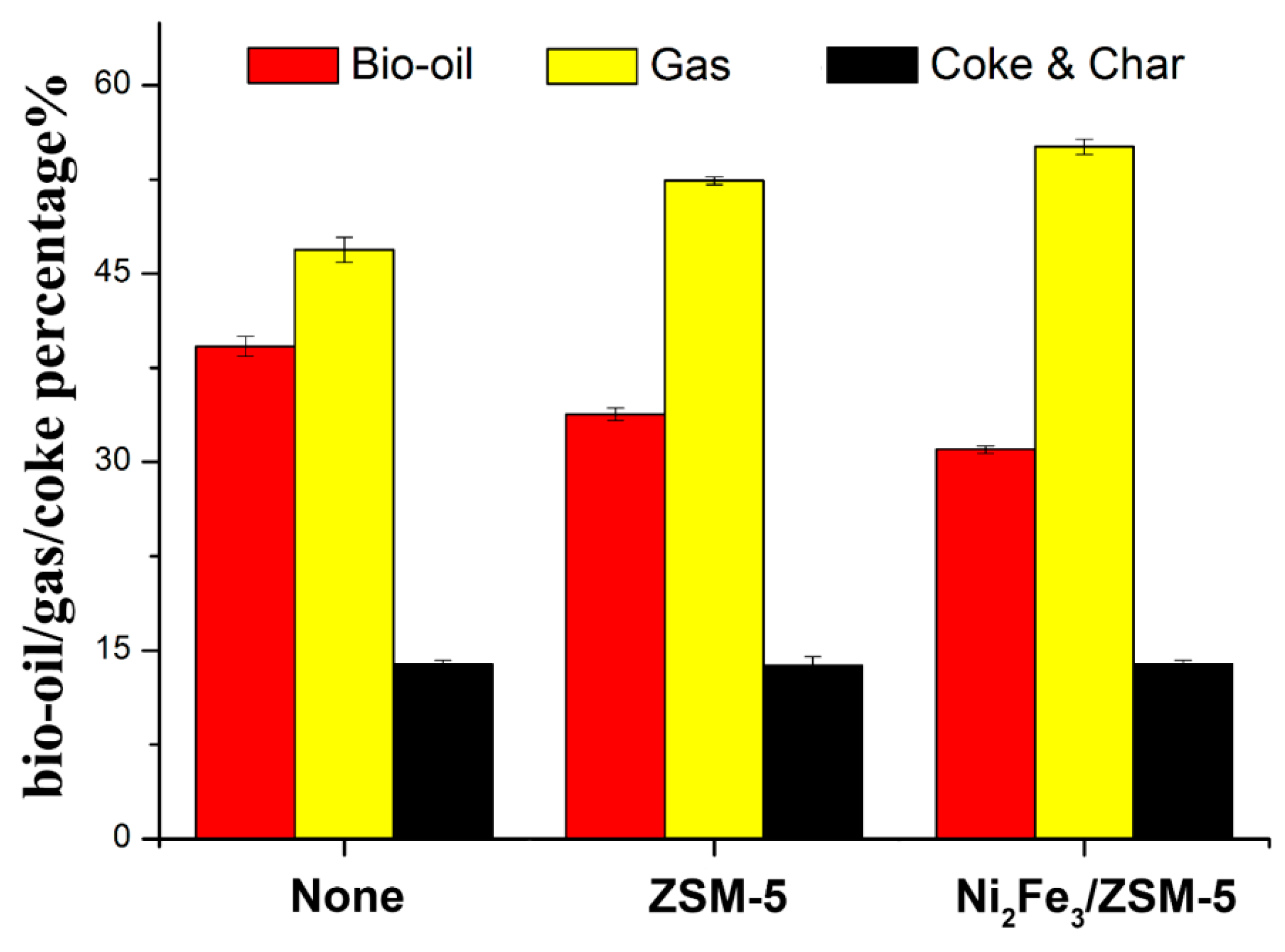



Catalysts Free Full Text Homogeneous And Heterogeneous Catalysis Impact On Pyrolyzed Cellulose To Produce Bio Oil Html




The Cheng Forum Chemical Engineering Forum Facebook
Heterogeneous catalysts are chemical catalysts whose physical phase is different from the physical phase of the reactants and/or products that take part in the catalyzed chemical reaction Typically, solid phase heterogeneous catalysts are employed in order to facilitate the chemical reaction between two gaseous reactants Special Issue "Biomass Derived Heterogeneous and Homogeneous Catalysts" A special issue of Catalysts (ISSN ) This special issue belongs to the section " Biomass Catalysis " Deadline for manuscript submissions closed (30 June ) Printed Edition Available! Synthetic catalysts generally fall into two separate groups a heterogenous or homogenous catalyst Heterogenous catalyst A heterogenous catalyst is a catalyst which is in a different phase to the reactants The most common form of a heterogenous catalyst is a solid catalyst that catalyses a reaction of either a liquid or gas that adsorbs to the surface




1 Schematic Diagram Of The Reaction Pathes In Homogeneous Download Scientific Diagram



Homogeneous Versus Heterogeneous Catalysis Download Scientific Diagram
If a catalyst dissolves in the reaction medium we call it homogenous catalysis, and if the catalyst is at another stage it is called heterogeneous catalysis In addition to chemical catalysis (chemocatalysis) there is also biocatalysis, where products such as enzymes are used as a catalystHomogeneous gold catalysts have high activity, enantioselectivity, and wellcharacterized structures, but heterogeneous gold systems show easier separation of the catalyst from the products, possible catalyst recycling, and adaptation to continuous flow processesHomogeneous catalysts work better in lowtemperature conditions (less than 250 0 C) In which the reactants and catalyst are in a similar phase Example 2SO 2 (g) O 2 (g) —– No(g) → 2SO 3 (g) Heterogeneous catalysis Heterogeneous catalysts are catalytic compounds that are in a contradictory phase from that of the phase of the reaction combination




Homogeneous And Heterogeneous Catalysis W3spoint




Advantages And Disadvantages Of Homogeneous And Heterogeneous Catalysts Download Scientific Diagram
Heterogeneous catalysis in terms of catalytic effectiveness, cata lyst properties, and catalyst separation1 Homogeneous cataly sts offers improvedThe 19th International Symposium on Relations between Homogeneous and Heterogeneous Catalysis The ISHHC was inaugurated in Brussels (Belgium) in 1974, as the "International Symposium on the Relations between Heterogeneous and Homogeneous Catalytic Phenomena", and has gathered scientists, engineers and technologists from around the worldThe homogeneous catalyst does not produce radicals as compared to the heterogeneous catalyst, and moreover, the heterogeneous catalyst reaction requires a higher operating temperature Temperature, residence time, CH 4 /O 2 ratio, and OH concentration are the important process parameters, and by optimizing these, we can obtain maximum conversion yield
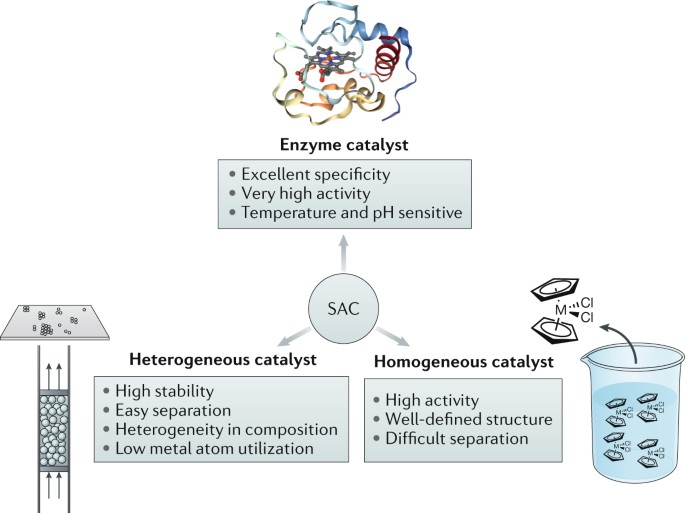



Heterogeneous Single Atom Catalysis Nature Reviews Chemistry
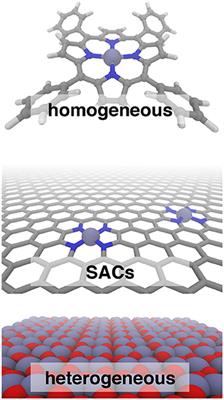



Frontiers Bridging The Homogeneous Heterogeneous Divide Modeling Spin For Reactivity In Single Atom Catalysis Chemistry
Interest in catalysis by metal nanoparticles (NPs) is increasing dramatically, as reflected by the large number of publications in the last five years This field, "semiheterogeneous catalysis", is at the frontier between homogeneous and heterogeneous catalysis, and progress has been made in the efficiency and selectivity of reactions and recovery and recyclability of the @article{osti_, title = {Single Atom Catalysis An Analogy between Heterogeneous and Homogeneous Catalysts}, author = {Yuk, Simuck F and Collinge, Gregory B and Nguyen, Manh Thuong and Lee, Mal Soon and Glezakou, VassilikiAlexandra and Rousseau, Roger J}, abstractNote = {In recent years, enormous efforts have been invested to improve the atomefficiency of metal catalystsV r d = 1 1 d i ν i mol s1 m3 General term for the reaction rate r =k(T)c A Terms for the reaction rate in homogeneous catalysis 2 or r =k(T)c A r =k(T)c A c B 1st order k s1 2nd order k m3 mol1 s1 k(T)=k 0 exp(−E A / RT) Concentrations are here only convenient as the reaction volume hardly changes How to describe the




Comparison Of Homogeneous And Heterogeneous Catalysts Download Scientific Diagram
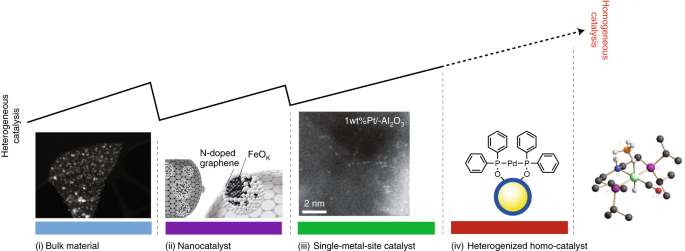



Bridging Homogeneous And Heterogeneous Catalysis By Heterogeneous Single Metal Site Catalysts Nature Catalysis
Reason Explained Homogenous are in the same phase as the reactants (so usually this will mean that they're liquid)Heterogenous are in a different phase than the reactants, are usually solidBoth increase the rate of reaction, are not consumed in the reaction is correct for What are the differences and similarities between homogenous and heterogenous catalysts?Homogeneous The separation of the products from the catalyst is generally expensive, the only exception being in biphasic catalysis Heterogeneous The separation of the products from the catalyst is usually straightforwardRecycling Homogeneous Recycling is expensive due to difficult treatment of the spent catalystThe catalyst is not behaving like a conventional homogeneous molecular catalyst but more like the metallic active sites exploited in heterogeneous catalysts 'We still get singlesite




Homogeneous And Heterogeneous Nickel Catalyzed Olefin Oligomerization Experimental Investigation For A Common Mechanistic Proposition And Catalyst Optimization Forget 17 Chemcatchem Wiley Online Library
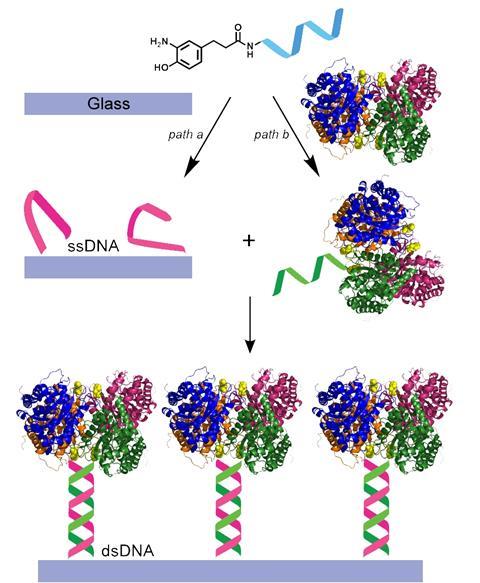



Combining Homogeneous And Heterogeneous Catalysis Feature Chemistry World
A homogeneous catalyst is a catalyst that is capable of dissolving in solution, because it by definition is in the same phase as the rest of the reactants in the solution Here are the principles of homogeneous catalysts that I see in my textbook (Inorganic Chemistry, Shriver, Atkins, Ch 25) PROS Homogeneous catalysts are effective at being highly selective towards Heterogeneous catalysis plays an important role in industrial chemical processing, fuel reforming, and energyproducing reactions Examples include the Haber–Bosch process, steam reforming, Ziegler–Natta polymerization, and hydrocarbon cracking (1 –8)Research in heterogeneous catalysis continues to flourish (9 –15) but iterative design and modification areHomogeneous catalysis catalysis in which the reactants and catalyst are in same phaseIe same physical state Example 2 S O 2 (g) O 2 (g) → N O (g a s) 2 S O 3 (g) Heterogeneous catalysis catalysis in which the reactants and catalyst are in phases Ie different physical states Example 2 S O ( g) O ( g) → P t (s) 2 S O 3 (g)




Single Atom Catalysis Bridging The Homo And Heterogeneous Catalysis Sciencedirect




A Review Of The Problem Of Distinguishing True Homogeneous Catalysis From Soluble Or Other Metal Particle Heterogeneous Catalysis Under Reducing Conditions Sciencedirect
A catalyst is a compound used to help a reaction occur faster by lowering the activation energy There are two types of catalysts, homogeneous and heterogeneous A homogeneous catalyst is aTypes of catalysis Catalysis of chemical reactions is generally divided into two categories Homogeneous Catalysis Homogeneous catalysis of chemical reactions is a process where the reactants involved in the reaction and the catalyst are in the same phase For example hydrolysis of sugar in the presence of sulphuric acidMajor differences between homogeneous and heterogeneous catalysts Homogeneous Heterogeneous Form Soluble metal complexes, usually mononuclear Metals, usually supported, or metal oxides Active site welldefined, discrete molecules poorly defined Phase Liquid Gas/solid Temperature Low (
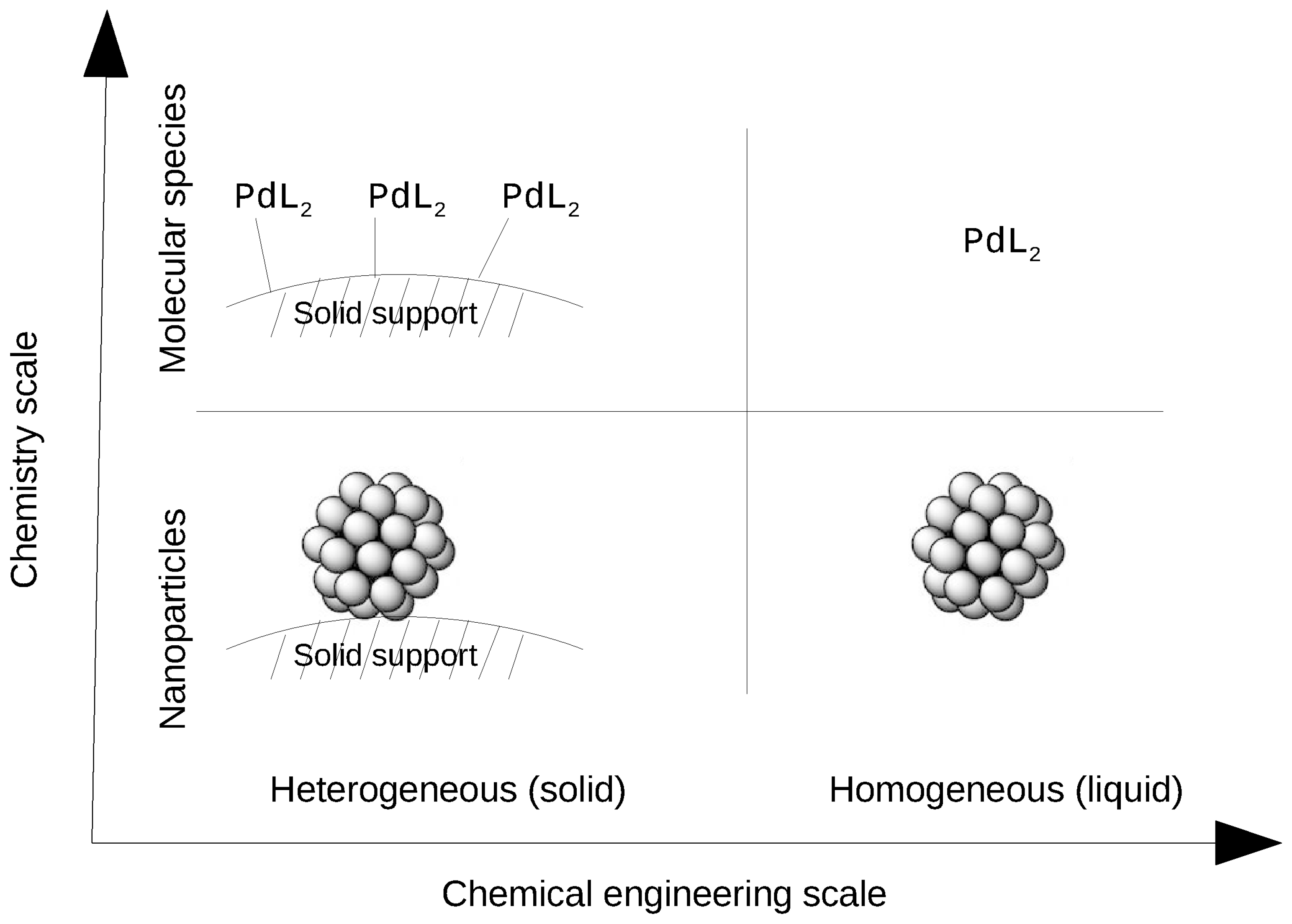



Catalysts Free Full Text About Solid Phase Vs Liquid Phase In Suzuki Miyaura Reaction Html




Homogeneous Catalyst An Overview Sciencedirect Topics
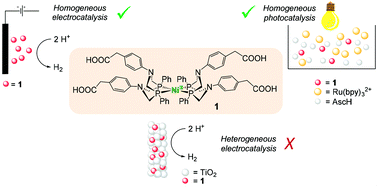



Homogeneous Vs Heterogeneous Catalysis For Hydrogen Evolution By A Nickel Ii Bis Diphosphine Complex Dalton Transactions Rsc Publishing




The Optimization Of Heterogeneous Catalytic Conditions In The Direct Alkylation Of Waste Vegetable Oil Royal Society Open Science




Heterogeneous Catalysis Wikiwand




Homogeneous Catalysis Wikipedia
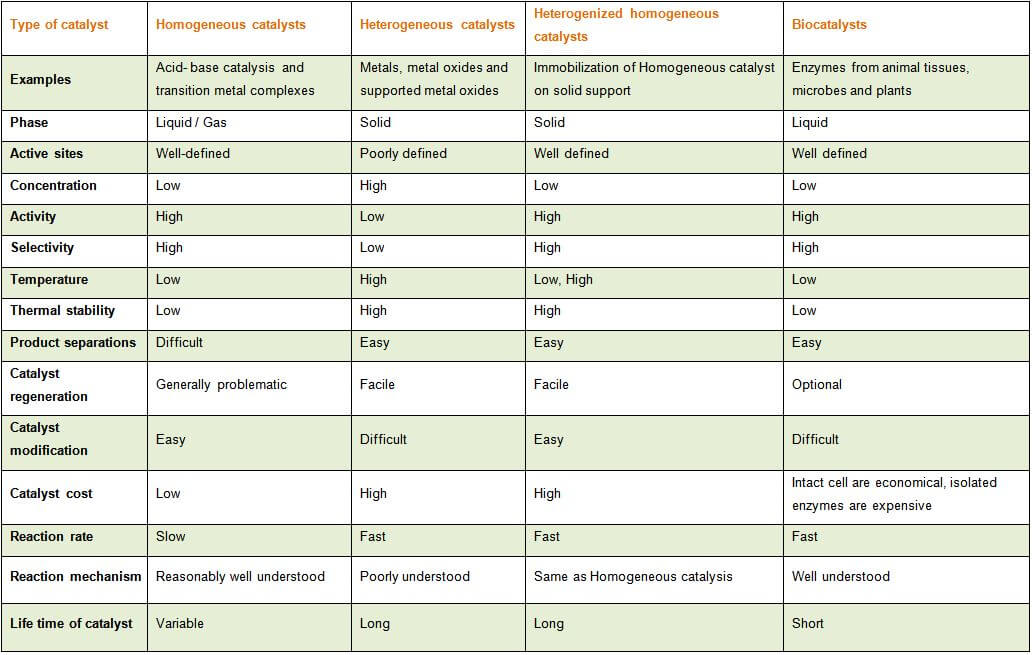



An Overview Of Different Types Of Catalysts Legal Advantage




Types Of Catalysis
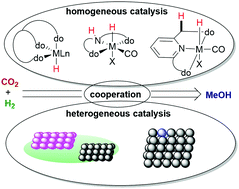



Homogeneous And Heterogeneous Catalysts For Hydrogenation Of Co2 To Methanol Under Mild Conditions Chemical Society Reviews Rsc Publishing



Homogeneous Vs Heterogeneous Catalysts Basic Introduction Youtube




C 4 1 Compare The Modes Of Action Of Homogeneous And Heterogeneous Catalysts Youtube




The Transformation Strategies Between Homogeneous And Heterogeneous Catalysts For The Coupling Reactions Of Co2 And Epoxides Olefins Sciencedirect
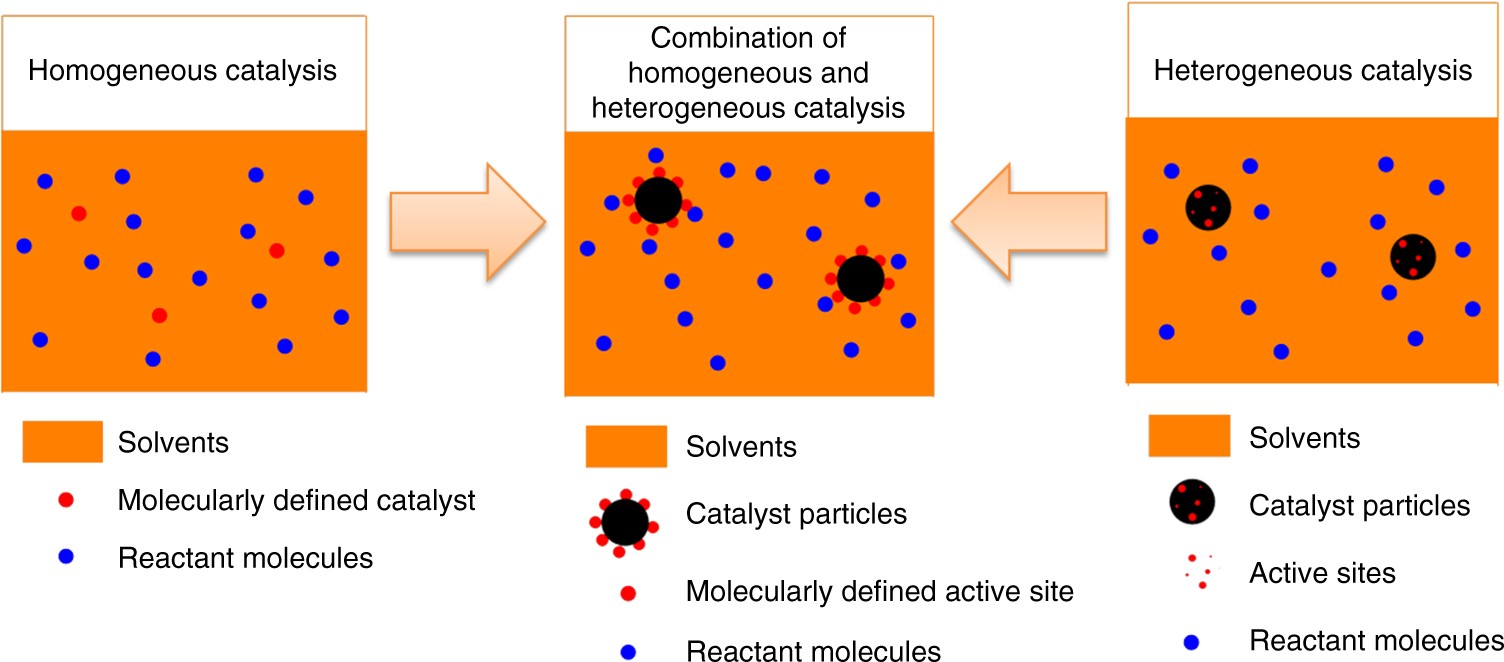



Synthesis Of A Molecularly Defined Single Active Site Heterogeneous Catalyst For Selective Oxidation Of N Heterocycles Nature Communications




Heterogeneous Catalysis Wikipedia



Http Www Ijetsr Com Images Short Pdf 1496 1500 Ieteb410 Ijetsr Pdf
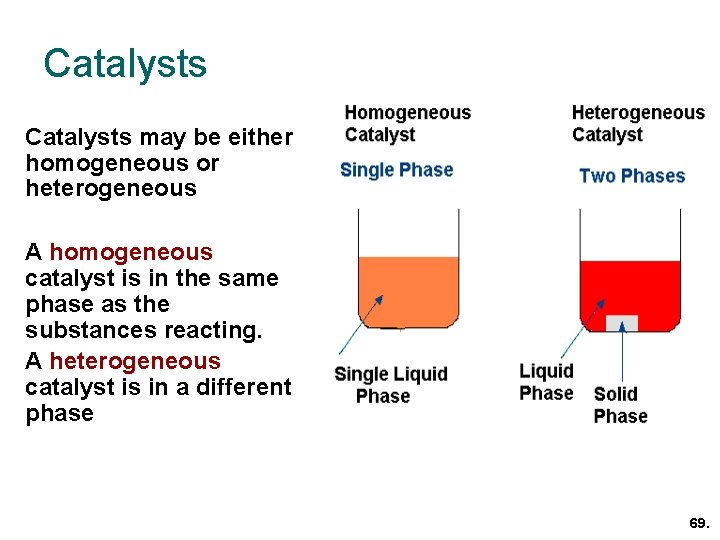



Chemical Kinetics Chapter 13 Topics 6 And 16
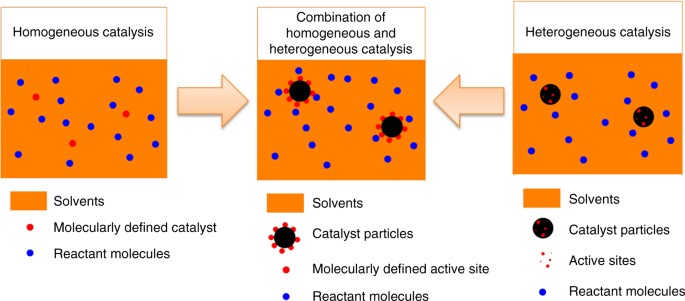



Synthesis Of A Molecularly Defined Single Active Site Heterogeneous Catalyst For Selective Oxidation Of N Heterocycles Nature Communications



Www Catalysis De Fileadmin User Upload Main Dateien 1 Forschung Fb Rosenthal Probevorlesung Hapke Hand Out Pdf




Differences Between Homogeneous And Heterogeneous Ef Processes Download Table
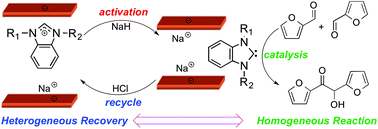



An Interchangeable Homogeneous Heterogeneous Catalyst System For Furfural Upgrading Green Chemistry Rsc Publishing



Www Mdpi Com 14 3049 23 7 1676 Pdf




Comparison Of Heterogeneous And Homogeneous Catalysis Download Table




Pdf Combining The Benefits Of Homogeneous And Heterogeneous Catalysis With Tunable Solvents And Nearcritical Water Semantic Scholar



Heterogeneous Catalysis And Catalyst Recycling All About Drugs
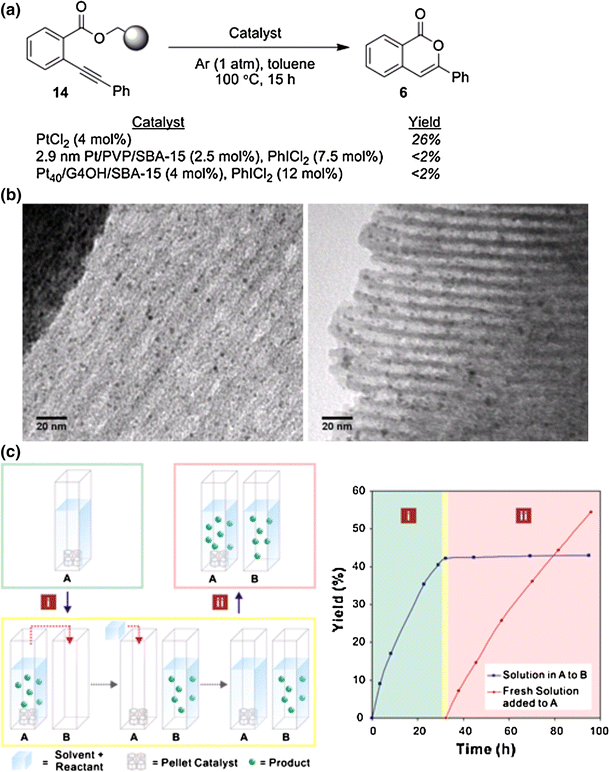



Polymer Encapsulated Metallic Nanoparticles As A Bridge Between Homogeneous And Heterogeneous Catalysis Springerlink



10 5 Catalytic Reaction Ppt Video Online Download




Homogeneous And Heterogeneous Catalysis Gagliardi Group



Catalyst In Acetylene Carbonylation From Homogeneous To Heterogeneous
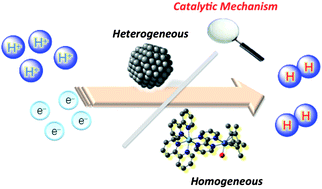



Catalytic Mechanisms Of Hydrogen Evolution With Homogeneous And Heterogeneous Catalysts Energy Environmental Science Rsc Publishing




Difference Between Homogeneous Catalysis And Heterogeneous Catalysis Surface Chemistry Youtube



Types Of Catalysis



Q Tbn And9gctrvbbisy7ikjyh35 8msk3jkudkzizuszlwrim5fn3f2i54ygk Usqp Cau



Homogeneous Catalyst Chander Jaswal Assistant Professor Chemistry Homogeneous



Homogeneous Catalysis Wikiwand



Catalysts In Industry




Asymmetric Heterogeneous Catalysis



1 A B 10 Red 18 Red 24



Www Ethz Ch Content Dam Ethz Special Interest Chab Icb Van Bokhoven Group Dam Coursework Catalysis 17 Homogeneous Heterogeoenous Catalysis Mesoporousmaterials Pdf




Catalysis Leibniz Institut Fur Katalyse Forschung Uber Katalysatoren Synergy Between Homogeneous And Heterogeneous Catalysis




Homogeneous Iron Catalysts In The Reaction Of Epoxides With Carbon Dioxide Della Monica 19 Advanced Synthesis Amp Catalysis Wiley Online Library



Immobilization Of Jacobsen Type Catalysts On Modified Silica



Types Of Catalysis
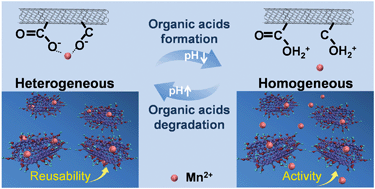



Performing Homogeneous Catalytic Ozonation Using Heterogeneous Mn2 Bonded Oxidized Carbon Nanotubes By Self Driven Ph Variation Induced Reversible Desorption And Adsorption Of Mn2 Environmental Science Nano Rsc Publishing
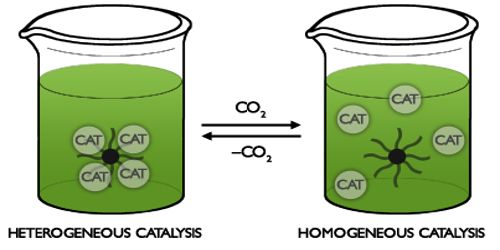



Homogeneous Catalysis Qs Study



Heterogeneous Catalysis Wikipedia
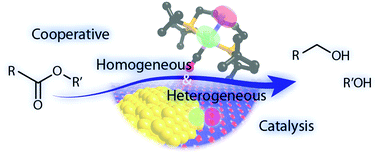



Heterogeneous And Homogeneous Catalysis For The Hydrogenation Of Carboxylic Acid Derivatives History Advances And Future Directions Chemical Society Reviews Rsc Publishing



Nanocatalysis For Green Chemistry Springerlink



Frontiers Heterogeneous Catalytic Conversion Of Sugars Into 2 5 Furandicarboxylic Acid Chemistry




Review Of Catalytic Transesterification Methods For Biodiesel Production Intechopen




Homogeneous Catalysis Wikipedia



How Can Heterogeneous Catalysts Differ From Homogeneous Catalysts Quora



Homogeneous Versus Heterogeneous Catalysis In Cu2o Nanoparticle Catalyzed C C Coupling Reactions Green Chemistry Rsc Publishing



1




Homogeneous Catalysis Catalysis Heterogeneous Catalysis




Metal Oxides In Heterogeneous Oxidation Catalysis State Of The Art And Challenges For A More Sustainable World Vedrine 19 Chemsuschem Wiley Online Library




Pdf Encyclopedia Of Life Support Systems Eolss Homogeneous And Heterogeneous Catalysis Semantic Scholar



Pubs Acs Org Doi Pdf 10 1021 Bk 13 1132 Ch001
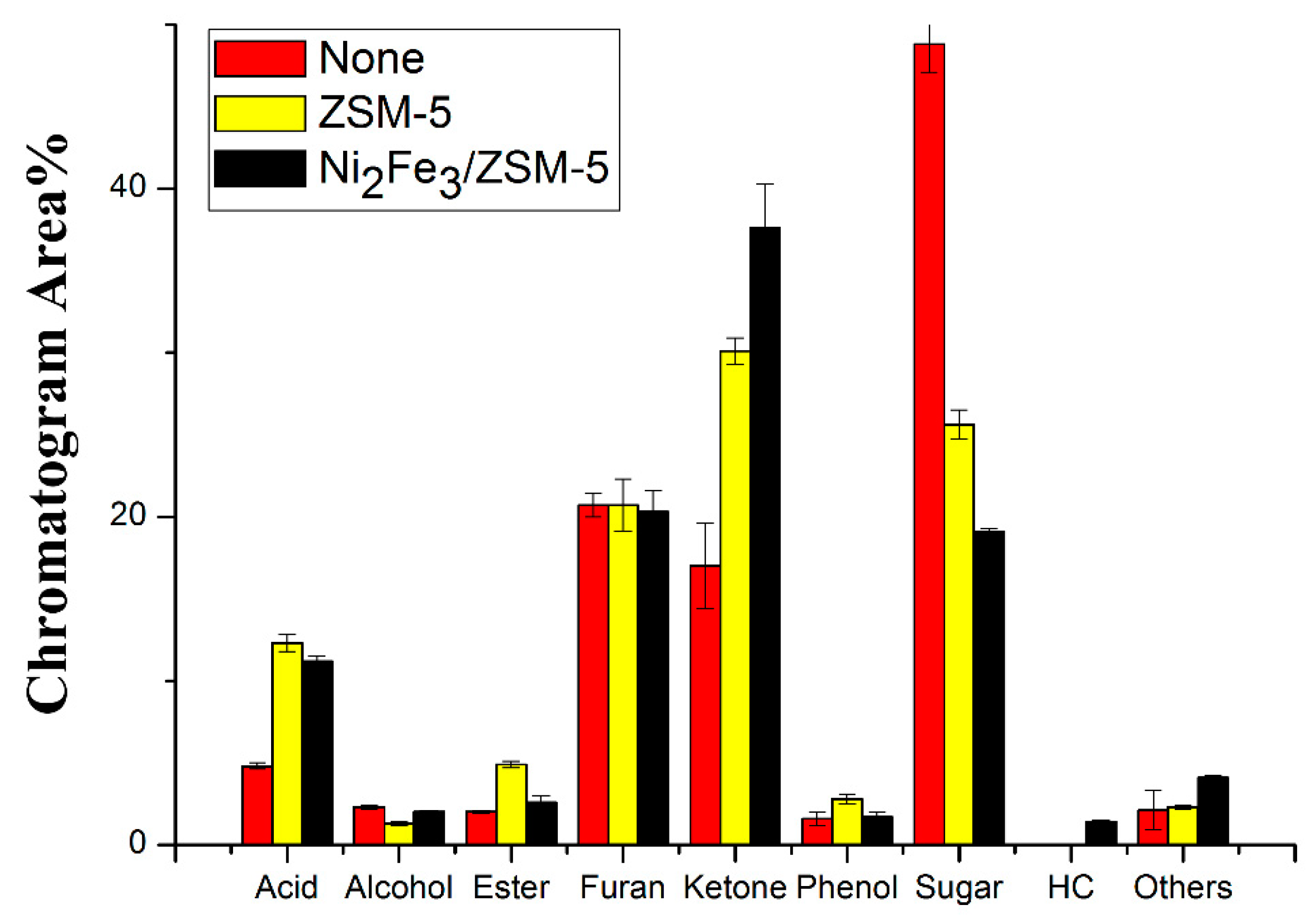



Catalysts Free Full Text Homogeneous And Heterogeneous Catalysis Impact On Pyrolyzed Cellulose To Produce Bio Oil Html



Heterogeneous Catalysis And Catalyst Recycling All About Drugs




Heterogeneous Catalysis Wikipedia




Explain The Difference Between A Homogeneous And Heterogeneous Catalyst Give An Example Of Each Youtube




Catalysis Mechanism Types Enzymes Biocatalysts Videos Examples
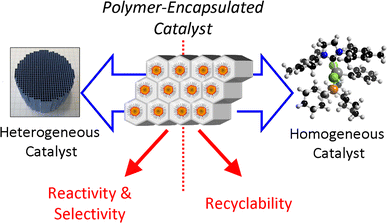



Polymer Encapsulated Metallic Nanoparticles As A Bridge Between Homogeneous And Heterogeneous Catalysis Springerlink




Navigating Through The Maze Of Homogeneous Catalyst Design With Machine Learning Trends In Chemistry
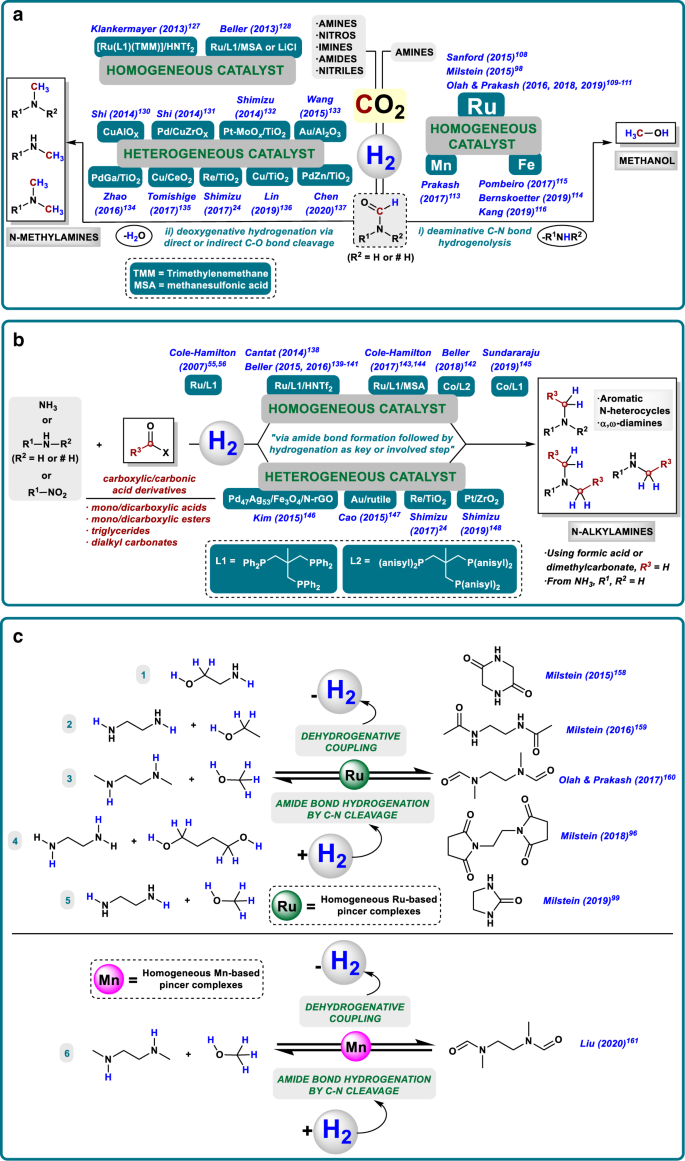



Homogeneous And Heterogeneous Catalytic Reduction Of Amides And Related Compounds Using Molecular Hydrogen Nature Communications




Single Atom Catalyst Based On Homogeneous Catalysis Prototype For Co2 Transformation




Catalysis Boundless Chemistry



Heterogeneous Catalysis All About Drugs
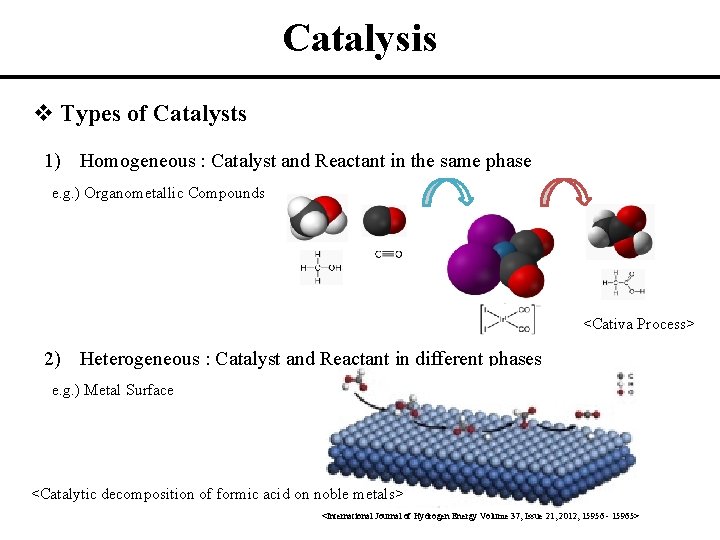



Heterogeneous Catalysis Solid State Physics 141 A Dohyung




Catalysis Boundless Chemistry




Navigating Through The Maze Of Homogeneous Catalyst Design With Machine Learning Trends In Chemistry
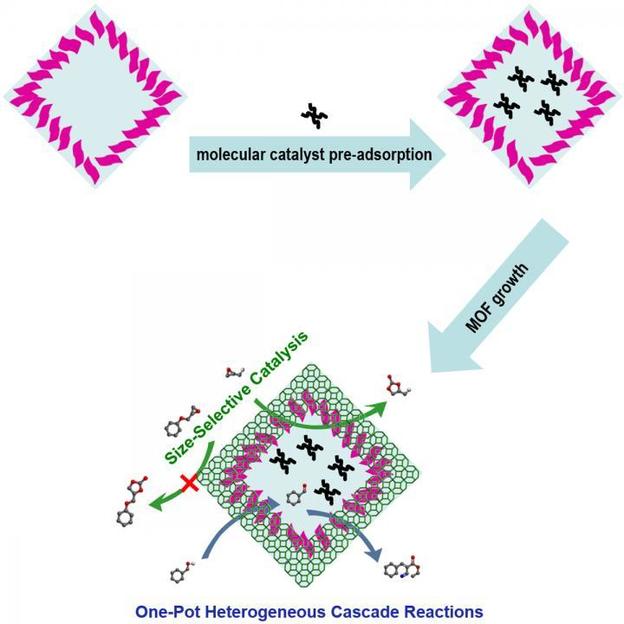



Lab In Hollow Mof Capsules Beyond Integration Of Homogeneous And Heterogeneous Catalysis




Navigating Through The Maze Of Homogeneous Catalyst Design With Machine Learning Trends In Chemistry



1



1




1 Homogeneous Vs Heterogeneous Catalysts Download Table



Nitrogen Reduction




Kinetics Theory Of Catalytic Mechanisms Heterogeneous Catalysis Homogeneous Catalyzed Reaction Examples Advanced A Level Gce Revision Notes




The Best Of Two Worlds From The Gold Catalysis Universe Making Homogeneous Heterogeneous De Almeida 12 Chemcatchem Wiley Online Library




4 Nitrophenol Reduction Catalysed By Au Ag Bimetallic Nanoparticles Supported On Ldh Homogeneous Vs Heterogeneous Catalysis Sciencedirect
コメント
コメントを投稿